At Clinical Trial Stage, Redesigned Neonatal Incubator
especiales
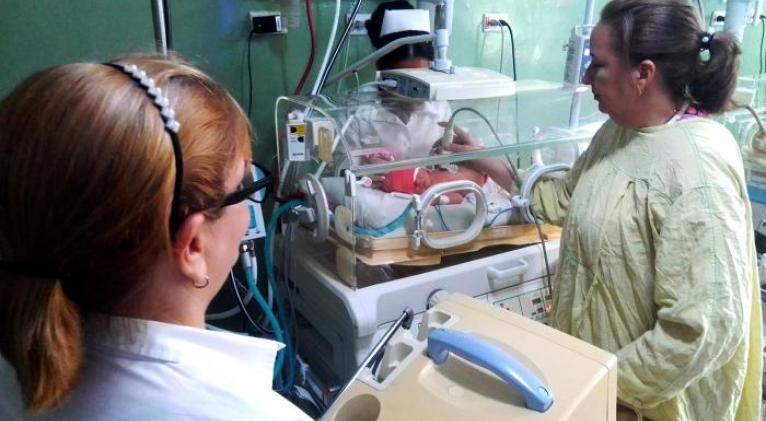
A new control for neonatal incubators of the V-2100G model, developed by the Technological Incubator of the Electronic Industry Group, at the request of the National Center for Electromedicine, is at its clinical trial phase.
Darien Piña Sánchez, director of the Technological Incubator, told Granma that it’s a device that no longer had technical support, due to the number of years of exploitation, so they got involved in developing a Cuban-based controller, which would allow revitalize it.
He explained that, for validation at the Center for State Control of Medicines, Equipment and Medical Devices (Cecmed, for its acronym in Spanish), a clinical trial is being carried out at Hijas de Galicia hospital, in the capital, where four incubators have been located.
He added that it’s a project that provides technological sovereignty, given that, in conditions of obsolescence, breakage or blockage, quick solutions can be given, as it’s an interface developed in the country.
Piña Sánchez specified that the Cuban Medical Equipment Company was the entity designated as manufacturer for the project, for which the technological transfer has already been made. He added that, initially, he was granted financing for the revitalization of ten of these instruments.
Regarding Medical Equipment Company productions, Lizbeth Vera García, Business Director of this industry, pointed out that, given the difficulties in acquiring raw materials for new productions, they have focused on the repair and maintenance of the equipments already in hospitals, these include Fowler beds and autoclaves, head tables and footstools, among others.
Translated by Amilkal Labañino / CubaSí Translation Staff














Add new comment