Cuba conducts phase III clinical trial for ataxia type two drug
especiales
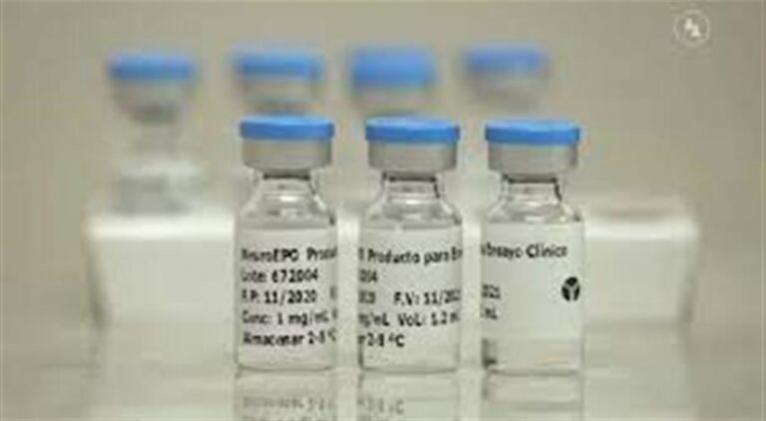
A phase III clinical trial to evaluate the efficacy and safety of the nasal administration of NeuroEPO in adult patients with spinocerebellar ataxias is being successfully developed in Cuba.
Cuba is the country in the world with the highest prevalence of Ataxia type sca2, at a rate of 36.2 cases per 100,000 inhabitants, although its frequency in the province of Holguin is 183 cases per 100,000 individuals, Granma newspaper reported.
Internationally, the incidence of this type of ataxia is three to five cases per 100,000 people.
Doctor of Science Luis Velázquez, founder of the Center for Research and Rehabilitation of Hereditary Ataxias (Cirah) in Holguín and president of the Cuban Academy of Sciences, reported that Phase III trial follows a Phase I/II one conducted between 2015 and 2016. The results of that study showed an improvement in cerebellar syndrome among treated patients and an improvement in their cognitive manifestations.
In addition, a high safety profile of NeuroEPO, a molecule developed by the Center for Molecular Immunology, which is being applied to different neurodegenerative diseases, was proven.
NeuroEPO in ataxia sca2. from preclinical studies to clinical trials in humans, was the focus of a meeting, this week, between scientists and health experts and the President of Cuba, Miguel Díaz-Canel, and Prime Minister, Manuel Marrero.












Add new comment