Soberana-Pediatrics clinical trial advances with the third dose in Cuba
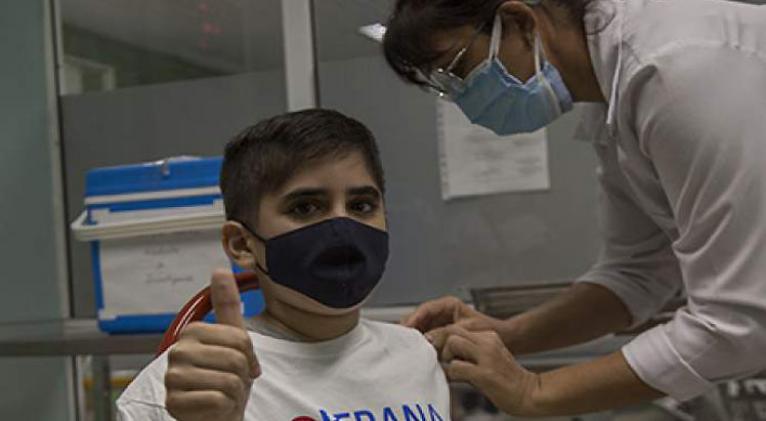
The first 25 teenagers included in Cuba's Soberana-Pediatrics anti-Covid-19 clinical trial received Monday the dose of the Soberana Plus vaccine candidate to conclude the vaccination scheme announced the leaders of the project.
This group of volunteers between 12 and 18 years of age has already received two doses of the Soberana 02 project, developed -as well as Soberana Plus- by the Finlay Vaccine Institute (IFV), the leading institution of the study.
In recent days, the administration of the second dose to the 12 to 18-year-old age group in the second stage of Soberana Pediatrics was completed and the three to 11 year-olds in Phase I of the trial.
The selection of the children under 12 was made after the safety of the first injection of Soberana 02 was proven in the adolescents, who had a medical follow-up of 24, 48, 72 hours, and one week after immunization.














Add new comment